I. Fluid and Electrolytes
- A. Water is the largest single component of the body
- B. 60% of the average adult’s body is fluid
- C. Homeostasis is the ability to maintain internal balance in the presence of external stressors
II. The Purpose of Body Fluids
A. Medium for transportation
- 1. Nutrients to cells
- 2. Wastes from cells
- 3. Hormones, enzymes, blood platelets, RBCs & WBCs
B. Cellular metabolism/chemical functioning
C. Normal body temperature
D. Facilitates digestion & elimination
E. Tissue lubricant
F. Solvent for electrolytes & nonelectrolytes
III. Body Fluids Overview
A. Intracellular ICF within the cells 40% body wtB. Extracellular ECF (20% body wt)
- 1. Intravascular IVF contained within blood vessels
- a. 5% body wt
- 2. Interstitial ISF surrounds cells, includes lymph
- a. 15% body wt
C. Transcellular TCF includes cerebrospinal, pericardial, pleural, synovial and intraocular fluids, sweat, + digestive secretions <1% body wt
IV. Composition of Body Fluids
A. Electrolytes
- 1. Ions
- a. Cations
- 1. Positively charged: sodium, potassium, calcium
- b. Anions
- 1. Negatively charged: chloride, bicarbonate, sulfate
V. Variations in Fluid Content
A. Age
- 1. Infant 77% water
- 2. Adult 60% water
- 3. Elderly 45% water
B. Gender
C. Body Mass
VI. Movement of Body Fluids
A. Osmosis
- 1. movement of a pure solvent through semipermeable membrane from area of low concentration to area of greater concentration
- B. Diffusion
- 1. movement of a solute in solution across a semipermeable membrane from area of high concentration to area of low concentration
C. Filtration
- 1. Process by which water & diffusible substances move together in response to fluid pressure; movement is from higher to lower pressure
D. Active Transport
- 1. Requires metabolic activity & expenditure of energy to move materials across cell membranes
VII. Osmosis
A. A shift of fluid from an area of low concentration to an area of higher concentration until the solutions are of equal concentration.
B. Membrane is permeable to the solvent, not the solute
C. Tonicity is the ability of solutes to cause osmotic driving forces
D. Moses moved water
VIII. Osmotic Pressure
A. Tonicity is the ability of solutes to cause osmotic driving forces
B. Exerted on permeable membranes by high molecular weight substances
C. To pull fluid across membranes
IX. Osmotic Pressure
A. A fluid with a high solute concentration has a high osmotic pressure
B. Of two solutions, higher osmotic pressure will draw fluid until equilibrium is reached
C. Osmotic pressure of a solution is called osmolarity
D. Osmolarity is measure used to evaluate serum and urine in clinical practice
E. Expressed in osmols, or milliosmols per kilogram (mOsm/kg)
F. Normal serum osmolarity is 280-295 mOsm/kg
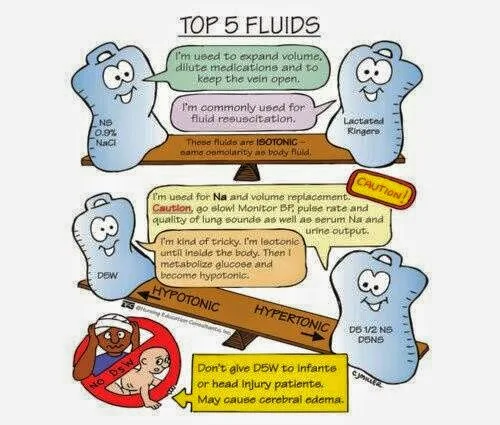
X. Types of Solutions
A. Isotonic – .9%/Normal Saline
- 1. Same concentration of solute as blood
- 2. Expands body’s fluids without causing fluid shift
B. Hypertonic (hyperosmolar) – D5NS, LR
- 1. Greater concentration of solutes than blood
- 2. Pulls fluids from cells causing them to shrink
C. Hypotonic (hypo-osmolar) – .45% NS
- 1. lesser concentration of solutes than blood
- 2. Moves fluid into the cells, causing enlargement
XI. Colloidal Osmotic/Oncotic Pressure
A. Affected by naturally produced serum proteins
B. Albumin exerts colloid pressure to keep fluid in the intravascular compartment by pulling water from the interstitial space back into the capillaries
XII. Diffusion
A. Is the natural tendency of a substance (or gas) to move from an area of higher concentration to one of lower concentration
B. This achieves balance
C. The difference between the two concentrations is called the concentration gradient
XIII. Facilitated Diffusion
A. Diffusion is facilitated by a carrier substance.
B. Facilitated diffusion requires a concentration gradient and sufficient carriers
C. Example: O2 & CO2 exchange
XIV. Filtration
A. Process of water and diffusible substances move together in response to fluid pressure
- 1. Called hydrostatic pressure
- 2. Determines the movement of water
B. Examples
- 1. Increased hydrostatic pressure on the venous side (congestive heart failure) water and electrolytes from the arterial capillary bed move to the interstitial fluid
- 2. Results in edema
A. Is necessary in the absence of concentration gradient or electrochemical gradient
B. Requires metabolic activity, expenditure of energy and, carrier substances to move materials across cell membranes
C. Allows cells to move molecules otherwise immovable – “uphill”
D. Examples
- 1. Na+ & K+ movement against the gradient
- 2. Mechanism that allows cells to absorb glucose & other substances to facilitate metabolic activities
XVI. Body Fluid Regulation
A. Homeostasis – physiological balance
B. Allows body to respond to disturbances in fluid & electrolyte levels to prevent & repair damage
C. Fluid intake
D. Hormonal regulation
E. Fluid output regulation
XVII. Cardiovascular System
A. Maintains:
- 1. Blood pressure
- 2. Cardiac output
- 3. Hydrostatic pressure
- 4. Adequate glomerular filtration rate
XVIII. Definitions
A. Osmolality
- 1. The concentration of osmotically active particles in solution expressed in terms of osmoles of solute per kilogram of solvent
B. Osmolarity
- 1. The concentration of osmotically active particles in solution expressed in terms of osmoles of solute per liter of solution
A. Regulated through the thirst mechanism located in the hypothalamus
B. Conscious desire for water
C. One of the major factors in fluid intake
D. Osmoreceptors monitor serum osmotic pressure; osmolality increases; hypothalamus is stimulated; thirst results
- 1. Increased osmolality – can occur with inability to take in fluids or administration of hypertonic fluids
- 2. Vomiting or hemorrhage: Hypovolemia; dehydration
XX. Hormonal Regulation (Endocrine)
A. Antidiuretic hormone (ADH)
- 1. Stored in posterior pituitary gland
- 2. Released in response to changes in blood osmolarity
B. Aldosterone
- 1. Released by adrenal cortex in response to increased plasma K+ levels or part of renin-angiotensin-aldosterone mechanism in hypovolemia
C. Renin
- 1. Proteolytic enzyme secreted by kidneys, responds to decreased renal perfusion 2nd to decrease extracellular volume
- 2. Triggers angiotensin I production converting to angiotensin II which causes massive selective vasoconstriction ultimately directing blood to perfuse kidneys
XXI. Fluid Output Regulation
A. Occurs through four organs of water loss
- 1. Kidneys
- a. Major regulatory organs of fluid balance; receive 180 L of plasma to filter & produce 1200 to 1500 ml of urine/day
- 2. Skin
- a. Regulated by the sympathetic nervous system; activates sweat glands
- b. Sensible – excess perspiration; can be observed
- c. Insensible – continuous; not perceived by the person; can increase significantly with fever or burns
- 3. Lungs
- a. Expire about 400 ml of water daily
- 4. Gastrointestinal tract (GI)
- a. 3 to 6 L of isotonic fluid moved into GI tract & returned to extracellular fluid daily
XXII. Regulation of Electrolytes
A. Cations
- 1. Sodium
- 2. Potassium
- 3. Calcium
- 4. Magnesium
B. Anions
- 1. Chloride
- 2. Bicarbonate
- 3. Phosphorus-phosphate
XXIII. Fluid Shifts
A. First Spacing
- 1. Normal fluid compartments
B. Second Spacing
- 1. Interstitial
C. Third Spacing
- 1. When fluid is trapped in a body space as a result of injury or disease,
- 2. Represents a fluid loss
- 3. Includes pericardial, pleural, peritoneal, joints
XXIV. Plasma to Interstitial
A. Pathology
- 1. + hydrostatic pressure
- 2. Decreased capillary osmotic pressure
- 3. + cap permeability
- 4. Obstructed lymph drainage
B. Manifestation
- 1. Edema, hypovolemia
C. Interventions
- 1. Fluid replacement
- 2. Monitor for overload
XXV. Interstitial to Plasma
A. Pathology
- 1. Decreased capillary permeability
- 2. Increased capillary osmotic pressure
B. Manifestations
- 1. Hypervolemia
C. Interventions
- 1. If normal, patient excretes fluid
- 2. With disease, must diurese
XXVI. Fluid Volume Deficit (FVD)
A. Thirst with 2% loss
B. Dry mucus membranes with 6% loss
C. Leads to cellular dehydration
D. Weight is best indicator
XXVII. FVD Causes
A. Anything causing loss of blood volume:
- 1. Blood loss
- 2. Polyuria
- 3. GI losses
- 4. Profuse diaphoresis
- 5. Third spacing
- 6. Decreased intake
XXVIII. FVD Manifestations
A. Hypotension
B. Increased pulse, respirations
C. Decreased urinary output, temperature
D. Poor skin turgor
E. Dry mucus membranes
F. Constipation
G. Increased hematocrit, plasma proteins, BUN, urine specific gravity
XXIX. FVD Interventions
A. Correct the underlying cause
B. Replace fluid depending on type lost
XXX. Fluid Volume Excess (FVE)A. Caused by fluid overload
B. Renal failure
C. CHF
D. Cirrhosis
E. Corticosteroids
F. Cushing’s syndrome
XXXI. FVE Manifestations
A. BP up, bounding pulse
B. Distended neck veins
C. Rapid wt gain
D. Peripheral edema
E. Pulmonary edema
F. CVP elevated
G. BUN decreased, hematocrit decreased
H. Plasma proteins decreased
XXXII. FVE Interventions
A. Treat the underlying cause
B. Fluid and or sodium restriction
C. Diuretics
D. Dialysis, paracentesis
XXXIII. Regulation of Acid-Base Balance
A. Metabolic processes maintain a steady balance between acids & bases
B. Arterial pH inversely proportioned to the hydrogen ion concentration
C. The greater the concentration, the more acidic the solution, the lower the pH & vice versa
D. The lower the concentration, the more alkaline the solution, the higher the pH
XXXIV. Chemical Regulation
A. Largest chemical buffer is carbonic acid & bicarbonate buffer system
B. Carbonic acid – carbon dioxide
C. Bicarbonate – excretion by the kidneys
XXXV. Biological Regulation
A. Occurs when hydrogen ions are absorbed or released by cells
B. Occurs after chemical buffering & takes 2 to 4 hours
C. Hydrogen must exchange with another positively charged ion
- 1. Chloride shift
- 2. Hemoglobin-oxyhemoglobin system
XXXVI. Physiological Regulation
A. Act as buffers (compensation) to return the pH to normal
B. Lungs
- 1. When the respiratory system is the problem agent – resp. acidosis; resp. alkalosis
- 2. Acts as the buffer when renal system is the problem
C. Kidneys
- 1. When the renal system is the problem agent – metabolic acidosis; metabolic alkalosis
- 2. Acts as the buffer when respiratory system is the problem
XXXVII. Disturbances in Electrolyte, Fluid, & Acid-Base Balance
A. Seldom occur alone
B. Can disrupt normal body processes
C. Each disturbance can cascade into a disturbance of the other
XXXVIII. Electrolyte Imbalances
A. Sodium
B. Potassium
C. Calcium
D. Magnesium
E. Chloride
XXXIX. Sodium
A. Hyponatremia
1. Net sodium loss or excess water excess2. Indicators & treatments depend on cause & ECF status
B. Hypernatremia
- 1. Excess water loss or sodium excess
- 2. Increases aldosterone secretion, sodium is retained, potassium is excreted
- 3. Body conserves fluid through renal reabsoption
XXL. Potassium
A. Normal amount is very small with little tolerance for fluctuation
B. Hypokalemia
- 1. One of most common electrolyte imbalances
- 2. Inadequate amount of potassium circulates in the ECF
- 3. Severe cases can affect cardiac conduction and function; most common cause is use of potassium-wasting diuretics
C. Hyperkalemia
- 1. Produces marked cardiac conduction abnormalities
- 2. Primary cause is renal failure; also seen in crushing injuries
Calcium
A. Hypocalcemia
- 1. Results from severe illnesses, especially ones that directly affect thyroid & parathyroid glands; renal insufficiency (inability to excrete phosphorous, as phosphorous rises, calcium declines)
- 2. S/S related to neuromuscular, cardiac, & renal systems
B. Hypercalcemia
- 1. Frequently a symptom of underlying disease resulting in excess bone reabsorption
Magnesium
A. Symptoms are a result of changes in neuromuscular excitability
B. Hypomagnasemia
- 1. Occurs with malnutrition, malabsorption disorders, & neuromuscular system disorders
C. Hypermagnasemia
- 1. Depresses skeletal muscles & nerve function
- 2. Depresses acetylcholine leads to sedative effect; leading to bradycardia, ECG changes, cardiac arrhythmias, decreased respiratory rate & depth
Chloride
A. Commonly associated with acid-base imbalance
B. Hypocloremia
- 1. Occurs with vomiting or prolonged NG tube or fistula drainage with loss of hydrochloric acid
- 2. Use of loop & thiazide diuretics
- 3. Can result in metabolic alkalosis
C. Hypercloremia
- 1. Occurs when serum bicarbonate falls or sodium level rises
Fluid Disturbances
A. Isotonic
- 1. Occur when water & electrolytes are gained or lost in equal proportions
B. Osmolar
- 1. Occur with losses or excesses of only water so that concentration of serum is affected
Acid-Base Balance
A. Arterial blood gases (ABG) are the best way to evaluate
B. Involves analysis of 6 components
- 1. pH
- 2. PaCO2
- 3. PaO2
- 4. Oxygen saturation
- 5. Base excess
- 6. Bicarbonate (HCO3)
C. Deviation from a normal value indicates imbalanc
PH
A. Measures hydrogen ion concentration in body fluids
B. Even slight changes can be potentially life threatening
C. Normal ranges 7.35 to 7.45
D. RULER OF THE BODY
PaCO2
A. Partial pressure of carbon dioxide
B. Reflects depth of pulmonary ventilation
C. Normal range 35 to 45 mm Hg
D. Indicates the concentration of CO2 in the blood
PaO2
A. Partial pressure of oxygen
B. No primary role in acid-base balance regulation if within normal limits
C. Lower levels < 60mm Hg can lead to anaerobic metabolism, resulting in lactic acid production & metabolic acidosis
D. Normal range is 80-100 mm Hg
Oxygen Saturation
A. Point at which hemoglobin is saturated by O2
B. Can be affected by changes in temparture, pH, & PaCO2
C. Normal range is 95% to 99%
Base Excess
A. Amount of blood buffer that exists
B. High value indicates alkalosis
- 1. Can result from sodium bicarbonate, citrate excess with blood transfusions, IV infusions of sodium bicarbonate to correct ketoacidosis
C. Low value indicates acidosis
- 1. Can result from elimination of too many bicarbonate ions, i.e. diarrhea
Bicarbonate (HCO3)
A. Major renal component of acid-base balance
B. Excreted & reproduced by the kidneys to maintain normal acid-base environment
C. Normal range is 22 to 26 mEq/L (this may vary slightly from lab to lab)
D. Indicator of metabolic cause of acidosis or alkalosis
E. Is buffer agent in respiratory acidosis or alkalosis
Types of Acid-Base Imbalances
A. Respiratory Acidosis
- 1. Excessive carbonic acid & increased hydrogen ion concentration (low pH)
B. Respiratory Alkalosis
- 1. Decreased carbonic acid & decreased hydrogen ion concentration (high pH)
C. Metabolic Acidosis
- 1. High acid content in blood causing loss of HCO3
- 2. Analysis of serum electrolytes to detect anoin gap (which reflects unmeasurable anoins)
D. Metabolic Alkalosis
- 1. Heavy loss of acid or increased HCO3 content in blood
- 2. Most common cause is gastric suction
Nursing Knowledge
A. Imbalances can occur at any age
B. Body’s adaptive compensatory mechanisms fail to maintain balance adequately
C. Health becomes compromised
D. Severity & long-term effects can influence client’s ability to return to optimal health
E. Prolonged compromise can lead to irreversible chronic health problems that affect the quality of life for client & family
Nursing Process – Assessment
A. Nursing history
- 1. Client’s history determines goals
- 2. Age of client is important factor
B. Prior medical history
- 1. Acute illnesses
- a. Surgeries, burns, respiratory disorders, head injuries
- 2. Chronic illnesses
- a. Cancer, cardiovascular disease, renal disease, GI disturbances
- 3. Environmental factors
- a. Exercise, exposure to temperature extremes
- 4. Diet
- a. Intake of fluids, changes in appetite, metabolic interference, physical problems
- 5. Lifestyle
- a. Smoking, alcohol use/abuse, drugs
- 6. Medication
- a. Prescription or OTC meds that perpetuate fluid imbalances
Physical Assessment
A. F&E/A-B imbalances can affect all systems
B. Thorough exam
C. Include client’s intake/output history, genitourinary patterns/changes
D. Lab study results
E. Client expectations
Nursing Process – Nursing Diagnoses
A. Actual
- 1. Ineffective breathing patterns
- 2. Decreased cardiac output
- 3. Deficient fluid volume
- 4. Excess fluid volume
- 5. Impaired gas exchange
- 6. Impaired mobility
- 7. Impaired tissue integrity/perfusion
B. Risks
- 1. Imbalanced body temperature
- 2. Deficient fluid volume
- 3. Impaired skin integrity/perfusion
Nursing Process – Planning
A. Goal & Outcomes
- 1. Client free of complication
- 2. Demonstrates fluid balance
- 3. Labs in range
B. Setting Priorities
- 1. Client’s condition sets priorities of ND
C. Continuity of Care
- y1. Discharge planning should happen early
Nursing Process – Implementation
A. Health promotion
- 1. Teach client & family to recognize risk factors
- a. Age, activities, specific chronic illness affects
B. Acute care
- 1. Daily weights
- 2. Enteral replacement of fluids
- 3. Fluid restrictions
- 4. Parenteral fluid & electrolyte replacement
- 5. IV therapy implications & risks
Complications of IV Fluid Administration
A. Systemic:
- 1. Fluid Overload
- 2. Air Embolism
- 3. Septicemia
B. Local:
- 1. Infiltration and Extravasation
- 2. Phlebitis
- 3. Thrombophlebitis
- 4. Hematoma
- 5. Clotting and obstructing
Fluid Overload
A. Fluid Overload: too much fluid in circulatory system: Rapid infusion, underlying cardiac, liver, or renal disease
B. S+S: moist crackles, edema, wt gain, dyspnea, shallow rapid resp
C. Treatment: Decrease flow rate, monitor VS + BS, position High Fowlers
D. Prevent: IV pumps, careful assessment
E. Complications: CHF, Pulmonary edema
Air Embolism
A. Occurs when air enters the bloodstream.
B. Is threatening when the air displaces blood to the cells
C. Most often associated with central veins
D. S+S; dyspnea, cyanosis, hypotension, weak, rapid pulse, loss of consciousness, chest shoulder, low back pain. Complication: death
E. Treatment
- 1. Clamp iv lines;
- 2. L trendelenberg position;
F. Prevention
- 1. Careful filling of IV tubes and syringes;
- 2. LuerLock adapters;
- 3. Air sensing pumps
Septicemia
A. Infection disseminated through the blood stream
B. Cause:
- 1. Contaminated IV products, break in technique
C. Risk: immunocompromised
D. S+S:
- 1. Elevated Temp, backache, headache, pulse and resp elev.
E. Treatment:
- 1. Symptomatic, local or systemic
F. Prevention:
- 1. Critical hygiene and technique
Infiltration
A. Infiltration:
- 1. Introduction of a non vesicant solution into surrounding tissues
- 2. Extravasation: Introduction of a vesicant or irritating solution into surrounding tissues.
B. S+S: edema, coolness, decreased flow, discomfort
C. Tx:
- 1. Discontinue, restart, compress with warm or cold to affected site, elevate
D. Prevention:
- 1. Careful securing of IV tubing, limit mobility of limb with IV, frequent observation of site
Phlebitis & Thrombophlebitis
A. Phlebitis:
- 1. inflammation of a vein from chemical or mechanical irritation.
- 2. S+S: red, warm at site or along vein, pain, tenderness, swelling.
- 3. Prevention: (frequency parallels length of insertion), use good aseptic technique, observe frequently.
- 4. Tx: discontinue IV, warm moist compress, elevate
B. Thrombophlebitis:
- 1. presence of a clot in addition to phlebitis.
- 2. S+S: same + more discomfort, elevated WBC, immobility
- 3. Tx: same: Prevent: avoid trauma at insert.
Hematoma
A. Blood leaking into tissue.
B. From perforation of vein during insertion or later.
C. S+S: ecchymosis, swelling, leak blood
D. Tx: remove, pressure, +/- ice, heat.
E. Prevent: careful technique during insertion
Clotting and Obstruction
A. Occur from kinked tubing, slow infusion rate, failure to flush line, empty IV bag
B. S+S: decreased flow, blood backing up,
C. Tx: if clotted, restarting at a new site is necessary
Nursing Process – Evaluation
A. Client care
- 1. Client teaching
- 2. Recognition of signs/symptoms of imbalance
- 3. Consult physician to enhance care in chronic conditions
B. Client expectations
- 1. Evaluate the client’s perception of care and goals
- 2. Use client input to understand needs and expectations